Abstract
Introduction: Patients with transfusion-dependent β-thalassemia (TDT) have increased morbidity and mortality and reduced health-related quality of life (HRQoL) compared with the general population owing to the mental, physical, and time demands of chronic transfusions, iron chelation, and treatment. Betibeglogene autotemcel (beti-cel) is a one-time ex vivo gene therapy for TDT that enables stable production of functional adult hemoglobin (Hb) sufficient for transfusion independence (TI). In 2 ongoing, fully enrolled, phase 3 studies, HGB-207 (Northstar-2; NCT02906202) and HGB-212 (Northstar-3; NCT03207009), 32/36 (89%) evaluable patients achieved TI (weighted average Hb ≥9 g/dL without packed red blood cell transfusions for ≥12 mo after drug product infusion) with a median (min-max) weighted average Hb of 11.52 (9.3-13.7) g/dL during TI (as of March 9, 2021). In the current analysis, we evaluated the effect of beti-cel on HRQoL in 12 adults and 18 pediatric/adolescent patients with TDT enrolled in phase 3 studies who achieved TI.
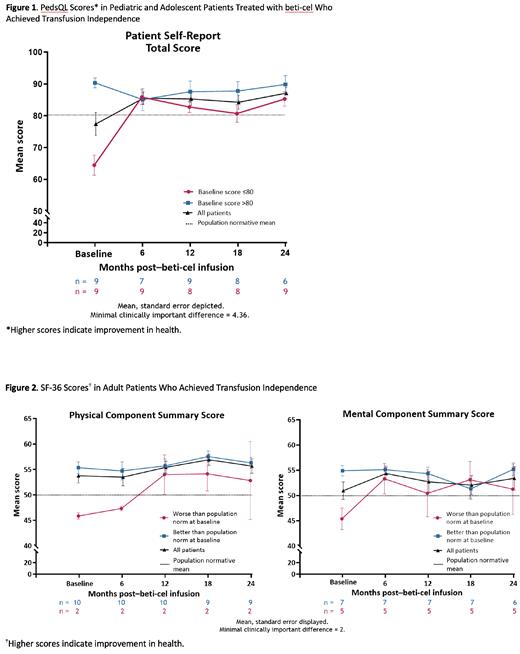
Methods: CD34+ cells were mobilized using granulocyte-colony stimulating factor and plerixafor, collected via apheresis, transduced with BB305 lentiviral vector, and infused back into patients after single-agent, pharmacokinetic-adjusted, busulfan-based myeloablation. HRQoL over time was assessed using the Pediatric Quality of Life Inventory (PedsQL; range, 0-100) for pediatric and adolescent patients (<18 years old); the EuroQol visual analog scale (EQ-5D-Y VAS; range, 0-100) for adolescent patients (11-17 years old); and the EuroQol (EQ-5D-3L; range, 0-1), Short Form-36 Health Survey Physical Component Summary and Mental Component Summary (SF-36 PCS and MCS, respectively; range, 0-100), and Functional Assessment of Cancer Therapy (FACT)-Bone Marrow Transplant and -General (FACT-BMT; range, 0-196 and FACT-G; range, 0-108) instruments for adults (≥18 years old). HRQoL was assessed at baseline and at Months 6, 12, 18, and 24 after beti-cel infusion. All instruments used Likert scales and evaluated physical, emotional, and social domains. Higher scores indicated improvement in HRQoL. Patients self-reported scores for all instruments, including the PedsQL. To assess clinical relevance of change in scores, minimal clinically important differences (MCID) of 4.36 for PedsQL and 2 for SF-36 were used.
Results: Forty-one patients were infused with beti-cel (HGB-207, n=23; HGB-212, n=18). Age at informed consent was 4-34 years; most patients (27/41, 66%) were <18 years old. Median (min-max) duration of hospitalization from conditioning through discharge was 44 (29-92) days. Among pediatric and adolescent patients with PedsQL data who achieved TI (n=18), the mean (SE) PedsQL total score (global population norm, 81.0) increased from 77.4 (3.6) at baseline to 85.3 (2.0) at Month 12 and 87.1 (1.8) at Month 24; these improvements were 1.8 and 2.2 times the MCID (4.36), respectively. Improvements were best for patients with scores below the population norm at baseline (Figure 1). Among adolescent patients with EQ-5D-Y data who achieved TI (n=12), the mean (SD) EQ-5D-Y VAS score increased from 81.4 (19.2) at baseline to 91.6 (4.9) at Month 12 and 92.4 (6.0) at Month 24. Among adults who achieved TI (n=12), mean (SE) SF-36 PCS and MCS scores (US general population norm, 50) increased from 53.8 (1.4) and 51.0 (1.7), respectively, at baseline to 55.4 (1.2) and 52.7 (2.0) at Month 12 and 55.7 (1.4) and 53.4 (2.3) at Month 24 (Figure 2). Increases in PCS and MCS scores at Month 24 were 1.0 and 1.2 times the MCID (2), respectively. Mean (SE) FACT-BMT and FACT-G scores in 11 adults increased from 125.8 (3.4) and 94.2 (2.6), respectively, at baseline to 128.4 (3.3) and 96.1 (2.5) at Month 12 and 128.9 (3.0) and 95.8 (2.1) at Month 24. Mean (SD) EQ-5D-3L Composite Index Score and VAS scores increased from 0.92 (0.15) and 85.2 (10.5), respectively, at baseline (n=12) to 0.96 (0.07) and 90.9 (4.5) at Month 12 (n=12) and 0.95 (0.08) and 94.2 (4.8) at Month 24 (n=11).
Conclusions: Beti-cel is potentially curative in patients with TDT through the achievement of TI and near-normal Hb levels. Beti-cel addresses the disease pathophysiology at a genetic level and allows patients to achieve TI. Pediatric and adult patients with TDT who achieved TI after receiving beti-cel in phase 3 studies showed early and sustained improvements in HRQoL, despite relatively high HRQoL with supportive care at baseline.
Kwiatkowski: bluebird bio,Inc.: Consultancy, Research Funding; Celgene: Consultancy; Sangamo: Research Funding; Apopharma: Research Funding; Bristol Myers Squibb: Consultancy; Imara: Consultancy, Research Funding; Agios: Consultancy; Silence Therapeutics: Consultancy; Bioverativ: Research Funding. Locatelli: Bellicum: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyl: Honoraria; bluebird bio, Inc.: Consultancy; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Walters: AllCells, Inc: Consultancy; BioLabs, Inc: Consultancy; Vertex pharmaceuticals: Consultancy; Ensoma, Inc.: Consultancy. Porter: Silence Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene (BMS): Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; bluebird bio, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; La Jolla Pharmaceuticals: Honoraria; Protagonism: Honoraria; Vifor: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Honoraria. Yannaki: Gilead: Speakers Bureau; SANDOZ: Speakers Bureau; bluebird bio, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Speakers Bureau. Kulozik: bluebird bio, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BioMedX: Consultancy, Honoraria. Thrasher: Rocket Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; 4Bio Capital: Consultancy, Membership on an entity's Board of Directors or advisory committees; Generation bio: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Orchard Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Thuret: bluebird bio, Inc.: Other: investigators for clinical trials, participation on scientific/medical advisory board; Apopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Other: investigators for clinical trials, participation on scientific/medical advisory board; Celgene: Other: investigators for clinical trials, participation on scientific/medical advisory board. Lal: Insight Magnetics: Research Funding; Protagonist Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; La Jolla Pharmaceutical Company: Research Funding; Chiesi: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; bluebird bio, Inc.: Research Funding; Agios Pharmaceuticals: Consultancy; Terumo Corporations: Research Funding. Guo: bluebird bio, Inc.: Current Employment, Other: Employee, Ownership Interest and Salary. Colvin: bluebird bio, Inc.: Current Employment, Other: Employee, Ownership Interest and Salary. Gruppioni: bluebird bio, Inc.: Current Employment, Other: Employee, Ownership Interest and Salary. Thompson: Baxalta: Research Funding; bluebird bio, Inc.: Consultancy, Research Funding; Biomarin: Research Funding; Celgene/BMS: Consultancy, Research Funding; CRISPR Therapeutics: Research Funding; Vertex: Research Funding; Editas: Research Funding; Graphite Bio: Research Funding; Novartis: Research Funding; Agios: Consultancy; Beam: Consultancy; Global Blood Therapeutics: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal